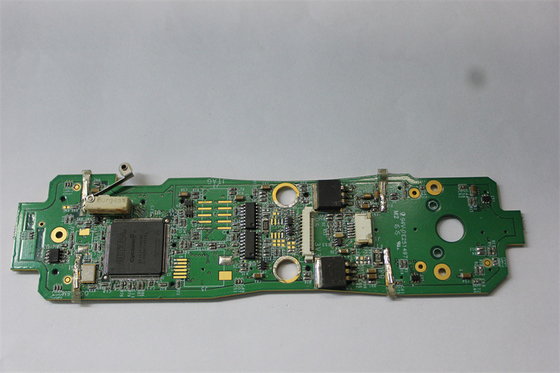
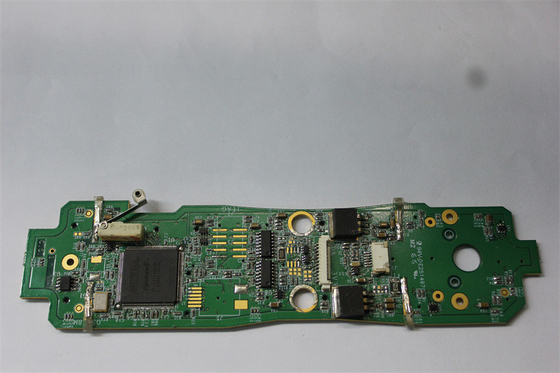
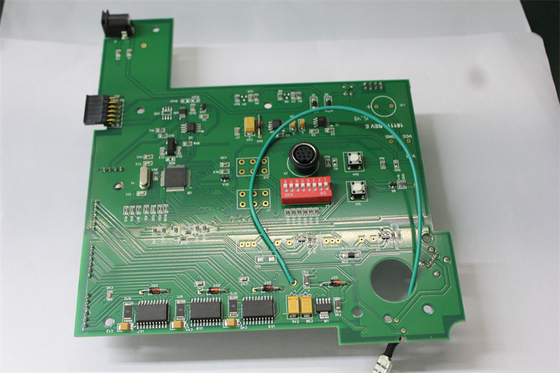
18um-30um Copper Oxygen Concentrator Medical PCBA ISO 13485
| Place of Origin | CHINA,VIETNAM,USA |
|---|---|
| Brand Name | IBE |
| Certification | IATF/TS16949,ISO13485,ISO 9001,ISO14001,UL |
| Model Number | IBE-1398745 |
| Minimum Order Quantity | no MOQ |
| Price | Negotiable |
| Packaging Details | ESD bag+card carton(custom made) |
| Delivery Time | 5-10 weeks depends on components lead time |
| Payment Terms | L/C, T/T |
| Supply Ability | 20000pcs+unit+month |
| Material | FR-4; High TG FR-4; Aluminum; CEM-1; CEM-3; Rogers, Etc | PCB Type | Rigid, Flexible, Rigid-flexible |
|---|---|---|---|
| Layer NO. | 1, 2, 4, 6, Up To 24 Layer | Copper Thickness | 0.5 OZ-3OZ (18 Um-385 Um) |
| Copper Plating Hole | 18um-30um | Min Trace Width | 0.075mm (3mil) |
| Min Space Width | 0.1mm (4 Mil) | ||
| High Light | 30um Copper Medical PCBA,oxygen concentrator pcb ISO 13485,Oxygen Concentrator Medical PCBA |
||
Customized Pcba Circuit Boards For Medical Oxygen Concentrator
IBE has been serving the contract manufacturing (OEM/EMS) industry nearly 20 years based in China. The service includes Electronic Design , PCB Fabrication, SMT and PCB Assembly, Components Sourcing, Prototyping, Box Build, and Turnkey Solution. To expand the business and provide better services to North American customers, IBE opened a new facility IBE (USA) in Fremont,CA since 2017, which can provide a full range of PCB assembly service. Also established the facility campus in Vietnam since 2020.IBE continues to invest in new technologies, providing a complete manufacturing solution to meet customers' needs and make the process more economical. Our major products are in markets of Smart Home Appliance,Smart Metering,Medical Facility,Automotive Electronic Device,Electromobile Power Charging Electronic Device, Digital Earphone,etc.
Medical devices have one crucial requirement - 100% reliability. When a patient’s health and life are at stake, nothing less than perfect functionality without failure is acceptable.
To achieve this level of reliability, your PCB manufacturer and assembly partner needs to meet and understand the various strict medical standards.
Medical standards have been in place for decades to guide the robustness and safety requirements of electronic designs. These standards are strict and can be challenging to meet so it’s important to verify your boardhouse’s credentials and protocols.
Certification:
Certified by ISO 9001 and 14001,IBE is a well-equipped and strictly managed manufacturer.We integrate our resources and manage production through MES system which can well optimize process and guarantee quality.Besides,IBE Group adheres strictly to the production and manufacturing standards of TS16949,automobile industry and ISO 13485,medical industry.
Production capability:
| 1 | Material | PR4,Halogen free, High TG,CEM3,PTFE,Aluminum BT,Rogers |
| 2 | Board Thickness | Mass Production:0.3-3.5mm Samples:0.21-6.0mm |
| 3 | Surface Finish | HASL,OSP,Immersion Silver/Gold/SN,Flash Gold, Gold Finger,Hard Gold Plating |
| 4 | PCB Panel Size | Max Mass Productoin: 610x460mm Sample:762x508mm |
| 5 | Layer | Mass production:2-58 Layers, Samples:1-64 Layers |
| 6 | Min. Drill Hole Size | Laser Drill 0.1mm,Machine Drill 0.2mm |
| 7 | PCBA QC | X-ray,AOI Test,Functional Test |
| 8 | Speciality | Automotive,Medical/Gaming/Smart Device,Computer,LED/Lighting,etc |
| 9 | Sanforized | Buried via, Blind via, Mixed Pressure,Embedded Resistance,Embedded Capacitance, Local Mixed Pressure, Local High Density, Back Drill, Impedance Control |
![]()
![]()
![]()